
Cardiovascular disease (CVD) is the leading cause of death in women. Ischaemia with non-obstructive coronary arteries (INOCA), a condition that mainly affects women, is associated with an increased risk of adverse events, including heart failure (HF), myocardial infarction, stroke and cardiovascular death.
INOCA is often unrecognised. It can be diagnosed by coronary angiography; one study showed that around 50% of women undergoing this invasive, ionising procedure had no angiographic evidence of obstructive coronary disease, compared with 7-17% of men. In the context of INOCA, cardiac magnetic resonance imaging (MRI) under stress is a class 2a recommendation, however, MRI under stress is rarely performed due to its cost, complexity and (lack of) reliability – image analysis remains qualitative most of the time.
Objectives and research hypothesis
The scientific objective of the SWEETHEART project is to provide a simple, cost-effective, non-invasive and non-ionising screening technique for INOCA that can be used for further human research.
SWEETHEART’s main contribution is the development of a portable electrocardiographic (ECG) mapping device designed to exploit the potential of MRI under stress. It is based on state-of-the-art flexible biosensitive sensors and signal processing. Several technological challenges are addressed in this project: high-sensitivity flexible textile sensors; motion-robust quantitative MRI under stress without the need for contrast agents; methodology demonstrating the MRI safety of portable devices.
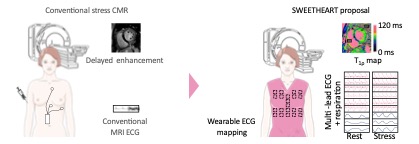
Epsidy’s industrial objective is to demonstrate the clinical effectiveness of this new approach to portable ECG mapping during cardiac MRI, and to bring this solution to market. Designed primarily for women, the proposed solution will also be applicable to men.
The industrial objective for Healtis is to adapt the methodology, and ultimately international standards, for assessing the MRI safety of active external/portable devices – current regulations are designed for implanted devices (ISO/TS 10974) – and to gain market share through this unique expertise.
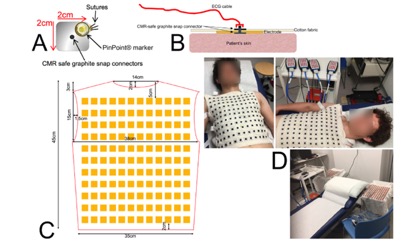
The ENSAIT/GEMTEX team has already tested the manufacture of the CMR-ECGI reusable dry textile electrode waistcoat, currently at technological maturity level 3 (TRL 3), with confirmed feasibility, reproducibility, scientific publications and patents pending. It is shown in Figures n and n+1. It contains 256 textile electrodes, 128 on the thorax and 128 on the back. The electrodes were designed and manufactured by embroidery on a ZSK industrial embroidery machine. Pilot data (n=500) already collected to date, at the University Hospital of London, using this prototype on 300 participants in a population-based study and 200 patients with cardiomyopathy, have confirmed the waistcoat’s durability and washability; its in vivo safety up to 3 T; and the high quality of unfiltered epicardial electrograms with minimal noise, regardless of body habitus. Wash tests were performed using an industrial washing machine on the e-textiles and associated electrodes to confirm the ECG signal quality retained after repeated use. Based on our pilot data, we anticipate the product’s resilience to more than 100 wash cycles.

Partners:
Financing : ANR
Start: 10/2023 – Stop: 10/2027
Contacts at Ensait / Gemtex: